
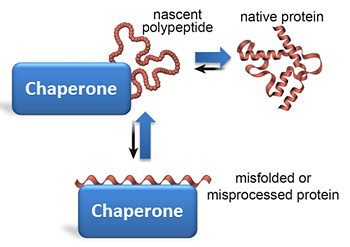
Moreover, folding intermediates expose hydrophobic surfaces that can engage in nonfunctional intermolecular interactions enabling aggregation (Fig. Off-pathway intermediates and kinetic traps slow folding, and non-native intramolecular interactions can lead to stably misfolded states ]. The folding free-energy landscape is rugged: Protein chains must traverse substantial energy barriers en route to the native state and consequently populate folding intermediates (Fig. Several factors complicate the folding process. Molecular chaperones inhibit aggregation, resolve kinetically trapped conformations, and provide kinetic assistance to folding by lowering free-energy barriers that separate folding intermediates from the native state. The accumulation of on- and off-pathway intermediates slows folding and entails the risk of misfolding into kinetically trapped states that are prone to form thermodynamically stable aggregates. During folding, proteins navigate a rugged, funnel-shaped potential free-energy surface en route to the native state. Molecular chaperones shape the energy landscape of protein folding. Current models are not generally predictive for protein folding pathways, even if substantial progress has been made toward prediction of protein folds ]. However, a unifying mechanism for protein folding remains elusive ]. The conformational search for the native state is thought to follow a funnel-shaped energy landscape, driven by the burial of hydrophobic residues, and the relative stability of native-like interactions that nucleate the folding reaction ] (Fig. Subsequent work over the last 50 years has provided detailed insight into the general principles that govern protein folding. Pioneering experiments by Anfinsen in the 1950s ] demonstrated that a small protein can fold spontaneously in the absence of additional factors in vitro. H/DX-MS, hydrogen/deuterium exchange mass spectrometry


 0 kommentar(er)
0 kommentar(er)
